Table of Contents
Washington University Experience | NEOPLASMS (EMBRYONAL) | Medulloblastoma, Histologically Defined | Medullomyoblastoma | 5G2 Medullomyoblastoma (Case 5) Myogenin 20X 1
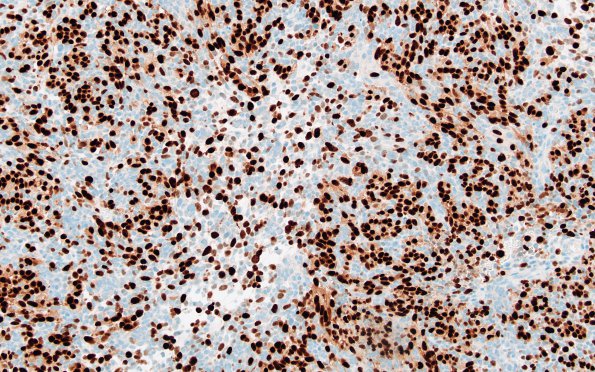
Myogenin transcription factor is strongly expressed in the strap and rhabdoid cells but much less in the primitive cells. (Myogenin IHC). ---- Not shown: Tumor cells show retention of nuclear expression of INI1 (encoded by SMARCB1) and BRG1 (encoded by SMARCA4). H3K36M, H3K27M and H3G34W mutations are negative in the tumor. H3K27Me3 nuclear expression is retained. Myoglobin is expressed in a smaller subset of tumor cells. TTF-1 is negative. Beta-catenin shows cytoplasmic reactivity without clear-cut nuclear staining. Repeat staining for beta-catenin confirmed the presence of immunoreactivity restricted to the cytoplasm. Neurofilament immunoreactivity is present in a smaller subset of tumor cells and also highlights both solid and infiltrative growth of the tumor. P53 highlights a few tumor cells. The Ki67 proliferation rate is estimated to be 50-60%. GAB1 shows very weak, patchy immunoreactivity in a subset of tumor cells. YAP1 is strongly positive in nearly all tumor cells. ---- Fluorescence in situ hybridization (FISH) shows no evidence of 17q, MYCN or MYC amplification. Per outside pathology report, ACVR1, NRAS, and FBXW7 mutations are found. Also by report, a ‘variant of unknown significance’ was found in SMARCA4, which encodes BRG1. ---- COMMENT: Diagnostic classification of this tumor is very challenging. Based on the tumor’s location in the pons and adjacent cerebellar peduncle and the extensive expression of glial and neuronal markers (GFAP, olig2, synaptophysin, and neurofilament), we favor a primary CNS origin for the tumor. The overall histologic appearance is that of a ‘small round blue cell’ tumor. In the posterior fossa of a child, the most common tumors with this appearance are medulloblastoma and atypical teratoid/rhabdoid tumor (ATRT). The tumor is reported to have a ‘variant of unknown significance (VUS)’ in SMARCA4, a gene known to be mutated in a rare subset of cases of ATRT. However, the retained expression of BRG1 (the protein encoded by SMARCA4) and INI1 in all tumor cells argues against ATRT. It is theoretically possible that this VUS in SMARCA4 is indeed pathogenic, but that there is persistent expression of the defective protein. However, myogenic differentiation is not typically seen in ATRT. This leads us to an alternative diagnostic possibility: medulloblastoma with myogenic differentiation (formerly known as medullomyoblastoma). Myogenic differentiation is well-documented in medulloblastomas, but it is a rare occurrence. How such differentiation affects medulloblastoma subclassification is uncertain. A caveat with respect to the diagnosis of medulloblastoma is that the location of the tumor outside of the cerebellum is unusual for this diagnostic entity. The location of the tumor may have influenced the differential diagnostic considerations entertained at St. Jude’s where the case was previously reviewed and the following diagnosis was rendered: “High-grade spindle cell sarcoma with rhabdomyoblastic differentiation”. Despite this outside diagnosis and for the reasons stated above, we favor a primary CNS origin for the tumor.